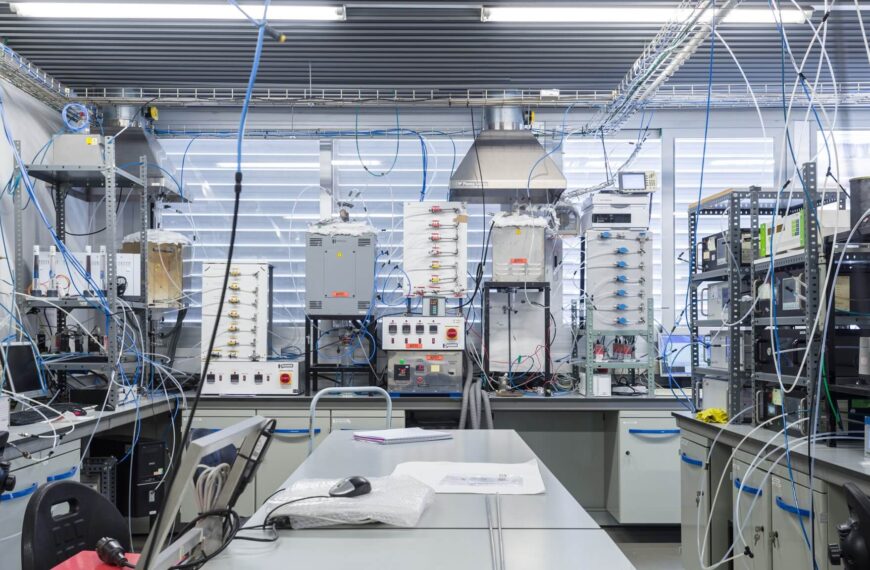
Welcome
The Thermochemical Processes Group, GPT, belongs to the Aragón Institute for Engineering Research (I3A) at Universidad de Zaragoza (Spain).
Both GPT and I3A have a strong focus on international collaboration and technology transfer, and are committed to deliver innovative science outcomes to society.
Its research groups work with groups and institutions around the world. It is one of the technology benchmarks in Aragón, performing research in the fields of Processes and Recycling, Industrial Technologies, Biomedical Engineering and Information & Communication Technologies.

Vision and Mission

Thermochemical processes are crucial for the efficient conversion and valorization of carbonaceous residual matter into valuable products and energy. Application of these processes can aid to solve some of the main technological challenges that humanity is facing in the XXI century: minimization of wastes and pollutants, renewable energy production and decarbonization towards climate change mitigation.
The Thermochemical Processes Group aims to help solving these challenges by applying a multidisciplinary, multiscale approach, with a strong focus on collaboration -both with other research groups and industry.
Brief history
The research activity in the Thermochemical Processes Group is, evidently, focused in the study of processes involving chemical reactions and temperature.
Our research activity in this field started in 1983 within the Chemical Engineering and Environmental Technologies Department at the School of Engineering and Architecture.
The first GPT studies were related to the thermal decomposition of lignocellulosic residues. Later on, relevant studies on biomass gasification were completed, including the scale-up to a pilot plant. In parallel, another research line was initiated, concerning the thermal degradation of several materials in air atmosphere or with different oxygen percentages. The latter evolved into different studies aiming at studying combustion kinetics in the gas phase, and elimination/reduction of pollutants and undesired species (NOx, hydrocarbons, etc.) in combustion reactions, including the development of kinetic models which explain the phenomena taking place.
Up to date, more than 300 articles on high-impact journals have been published as a result of our research. See our publications list to get a glimpse of all the research carried out in these 4 decades.

Strong focus on knowledge transfer

The group has always had very clear in mind that the research activity developed had to be oriented toward providing a benefit to society in terms of valorization of wastes and residues, which is our field of expertise.
Therefore, the ultimate aim of the work carried out by the GPT has always been to conduct the transfer of the scientific knowledge acquired, and of the technology developed, to companies and to the scientific community in order to make, on the one hand, biomass exploitation economically viable, and on the other hand, to contribute to the advance of the scientific knowledge in this field.